STARLIT
Aiming for ‘first-time-right’ treatment for cancer patients
- Project
start
October 2017 - Project
end
September 2020 - Project
leader
Frank van der Linden
Philips, the Netherlands - More
information
https://itea4.org/project/starlit.html
The global incidence rate of cancer is expected to grow by 70% over the next two decades, with Radiation Therapy (RT) treatment currently recommended for 52% of new patients. Although radiation oncology has caused a drop in mortality for several cancers, the need remains to reduce side effects such as incontinence or dysphagia. The solution lies in ‘first-time-right’ treatment in which the right dose is given to the tumour while keeping the dose to healthy tissue as low as possible to prevent side effects.
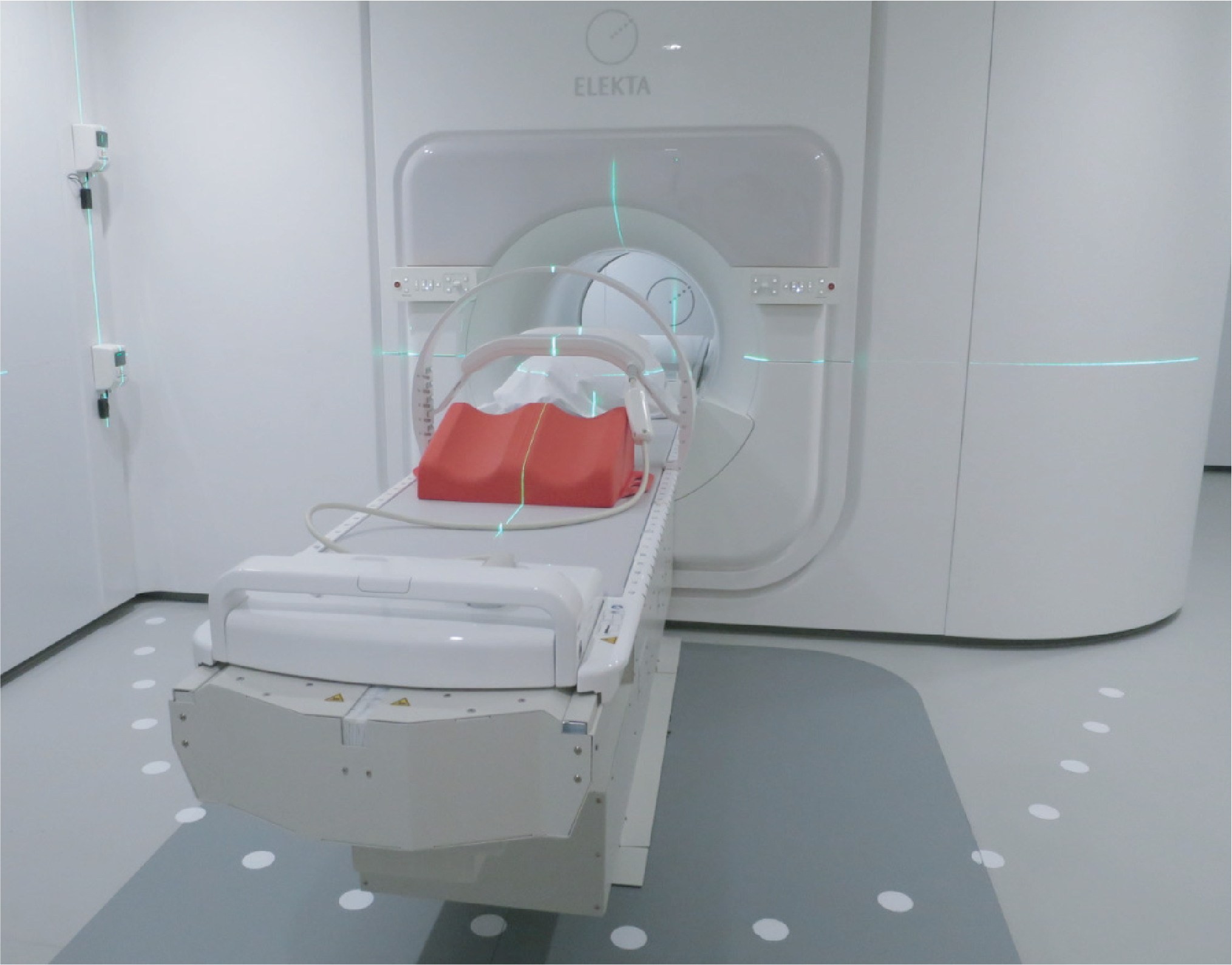
The ITEA project STARLIT - a follow-up to the award-winning ITEA project SoRTS - gathered 14 partners from Austria, Canada, the Netherlands and Sweden and developed technology to improve treatment accuracy and minimise the unintended dose in image-guided radiation therapy. A focus area for both projects was to improve the real-time connections within a system of systems called Elekta Unity, comprising a Philips MRI scanner for imaging and an Elekta linear accelerator for radiotherapy. STARLIT improves the results of SoRTS by reducing the latencies even more, allowing tumour movement to be followed with the treatment beam. In addition, during therapy, the delivered dose is continuously evaluated. First and foremost, this improves quality of life for cancer patients by reducing unintended side effects; it also provides greater efficiency in healthcare as a whole thanks to the reduced number of fractions.
Real-time adaption of cancer treatment
STARLIT has improved Elekta Unity’s software to speed up imaging, thereby allowing a number of intervention and monitoring processes to be carried out in real time. The STARLIT system is integrated with low-latency connections, causing minimal delay in the processing of computer data, and a feedback loop, allowing for real-time adaption of treatment based on separate monitoring processes. These verify that the delivered dose and the position of the tumour are correct.
The project’s main focus was technical feasibility, which resulted in several very successful outcomes. Before the project, for example, low-latency motion detection for tracking was clinically unpracticable or suffered delays of over 500 ms, whereas STARLIT has created a prototype with delays of just 200 ms. High-resolution imaging has also seen an enormous boost: an echo time reduction from over 100 ms to 70 ms, a 20-30% increase in signal-to-noise ratio (this ratio compares the level of a desired signal to the level of background noise) and a decrease in distortion from more than 10 mm to less than 1 mm. Thanks to these strong technical improvements, physicians can benefit from higher confidence and control of treatment delivery and potentially reduce margins while the patient, as a result of margin reductions, can potentially benefit from a risk reduction of side effects or tumour control in case the maximum dose is limited by the adjacent organs at risk, thus improving the overall safety of the treatment.
Personal treatment
Unfortunately, the project leader himself needed radiotherapy during the last year of the project. The traditional process would have involved 20 treatments over four weeks. However, the MR-LINAC treatment, based on the SoRTS project results, offered a treatment of five sessions over 20 days. This reduced the burden of travelling to the hospital substantially and the side effects, like fatigue, were also much less significant. As less tissue is damaged, the recovery time in general is also much shorter. In fact, he was even able to work about half of the time during these 20 days instead of being out of work for four weeks. Additionally, possible discomfort, like being partially out of action for a few months, did not occur with the MR-LINAC treatment.
As a result of the STARLIT project, the number of treatments will be reduced even further and so too will the side effects and burden for patients in the future. And in addition to the clear benefits that the MR-LINAC treatment offers patients, there is also a strong benefit for hospitals. As fewer treatments are needed per patient, they can treat many more patients in the same amount of time. In the abovementioned situation, for example, four patients can be treated instead of one in approximately the same timeslot.
Treatment of pancreatic cancer
The number of radiotherapy treatments will be reduced even further and so too will the side effects and burden for patients in the future.
In December 2022, a milestone was reached in radiation therapy as the first patients completed their full course of radiation therapy treatment with Elekta Unity using Comprehensive Motion Management (CMM) with True Tracking and automatic gating functionalities. A patient with pancreatic cancer was treated at University Medical Center Utrecht using Unity’s CMM developments. This first ever treatment delivery using CMM went smoothly and did not increase the total treatment time per fraction. The beam was automatically turned on when the patient was in the exhale phase of breathing, so they were not required to hold their breath for extended periods. Based on initial results, there is great potential in using Unity’s CMM functionality to treat abdominal cancers, lung tumours, prostate tumours – wherever there is motion, regardless of the cause. [1]
Strong complementarity
STARLIT’s commercial side has also seen great successes, helping to position Europe for dominance in the MR-guided RT market. The projected annual top-line revenues for the consortium are over USD 650 million after 2020. Real-time tracking is a fundamental step towards a 10% annual growth rate for this market as a whole, while the brachy market may also grow by 20%.
As for Elekta, updates have been done to the already successful Unity. A user group also exists for Unity, meeting one or two times a year to discuss improvements. This allows potential users, such as hospitals, to first test the system before buying it. Over 80 orders have already been placed in more than 25 countries
In addition, Philips had sold roughly 10,000 Compressed SENSE software licences by Q4 2022, which have been used in nearly 25 million examinations.
The STARLIT partners show a real complementarity in their collaboration, in which the involved SMEs have a unique role as the creators of additional products that improve STARLIT’s efficiency. For example, IT-V Medizintechnik from Austria created the Head & NeckSTEP M and the HeadSTEP MRL PushPIN, respectively the only head and neck positioning devices for iCAST and PushPIN masks officially certified for use with Elekta Unity. So far, a couple of devices have been sent for testing and demonstration and some of them have already been sold. IT-V expects a strong increase in sales numbers in the next years.
Tesla Dynamic Coils (TDC) from the Netherlands developed a head-andneck (H&N) coil for the Elekta Unity system. The outcome was a radiolucent flexible coil that demonstrated a threefold increase in signal-to-noise ratio (SNR, the quality indicator of MR images) compared to the traditional coil in the Elekta Unity and was also compatible with other Philips 1.5T systems, such as the PET MR and MR SIM. This breakthrough in technology inspired TDC to create multiple other coils with increased SNR that create a better fit due to the flexibility of the coil and, at the same time, reiiiduce the likelihood of claustrophobia for patients. Thanks to participation in the project, TDC was able to address a new market for flexible and wearable coils which increase the comfort for the patient, especially children. They were able to hire an engineer who specialises in flexible coils and are maturing the technology to TRL 8. Overall, the STARLIT consortium played a crucial role in driving TDC's progress forward.
Modus QA from Canada contributed to quality assurance during project testing with the Quasar MRI⁴D Motion Phantom, which is the world’s first MRsafe programmable motion phantom. The 4D motion phantom has a patented deformable tumour target, reduces measurement latency from over 50 ms to roughly 500 μs and improves target position precision from 1 mm to 0.25 mm. Modus QA has continued to develop the deformable tumour target with the goal of better addressing evolving market requirements. The product development process is nearing completion and Modus QA expects 4D MRI gating to be available on all MR-LINACS in the future.
Quantib from the Netherlands developed and improved upon their Visual Scoring Tool. By performing image quality assessments using the Visual Scoring Tool, image tuning parameters can be optimised, including image acquisition parameters between different imaging techniques. This tool has played an important role in image quality assessment for Quantib’s current line of products, including those for brain and prostate MRI. Using the tool, sub-optimal medical images, e.g. due to acquisition artifacts or obtained using poor imaging parameters, could be easily identified. By excluding these cases, Quantib ensured that only the highest quality images were used to develop novel algorithms, thereby improving algorithm performance. In addition to generating 19 fulltime positions within the consortium, STARLIT has led to eight Master theses, one PhD thesis and four new courses at Utrecht University. Combined with the 51 publications so far, the ongoing standardisation work of Philips and Elekta and the development of a 4D deformable QA/QC phantom platform by Modus QA, this will drive adoption of the project results to the benefit of patients across the globe.
Improved quality of life for patients
Promising uptake paves the way for STARLIT’s most important result: improved quality of life for patients, who currently go through the radiation procedure up to 20 times. Higher doses with greater accuracy could reduce this to two or three times – perhaps even just once. This means less travel to hospital and potentially fewer side effects. Philips plans to extend its image-guidance MRI technology to other treatments, such as cardiac catheter intervention, oncological ablations and neuromodulation, cascading STARLIT’s technology throughout the healthcare system.

Other chapters
Use the arrows to view more chapters

Editorial
By Jan Jonker

25 years of ITEA
Success built on the inheritance of a very strong base

Country Focus: Germany
Driven by digitalisation, supported by networks

Software AG
A one-stop shop where innovation is crucial

ITEA Success story: PARFAIT
Connecting IoT with greater security

IVVES Project benefits story
Create higher value thanks to automated ESG score calculation

Community Talk with Alain Coulombe
Disruptive entrepreneur to the core

25 years of ITEA
Ground-breaking innovations we’re still grateful for today

By and for end-users
Junkkari brings predictive maintenance to unchartered domains

ITEA Success story: STARLIT
Aiming for ‘first-time-right’ treatment for cancer patients

The Eureka Clusters Sustainability Call 2022 projects
Innovations making industry more sustainable and greener

SME in the Spotlight: The REUSE Company
Constant evolution in systems engineering

