SIGNET opens interfaces to MRI scanner for enhanced image-guided treatment
Magnetic resonance imaging (MRI) has expanded beyond diagnosis and is becoming an essential part of certain medical treatments. For example, by measuring the temperature during ablation or locating treatment devices, like dedicated MR-compatible catheters in the heart. Over the past decades, many treatment devices have been integrated with the MRI system in different ways and with multiple technical interfaces, hampering innovation speed, maintainability and extendibility.
The ITEA project SIGNET aims to address these challenges by integrating diverse treatment devices with differing unique needs via a single technical interface to the Philips MRI system. Named COMRADE, this interface has been developed in cooperation with consortium partners Brain Science Tools, IMRICOR, MACHNET, Galgo Medical and UMC Utrecht.
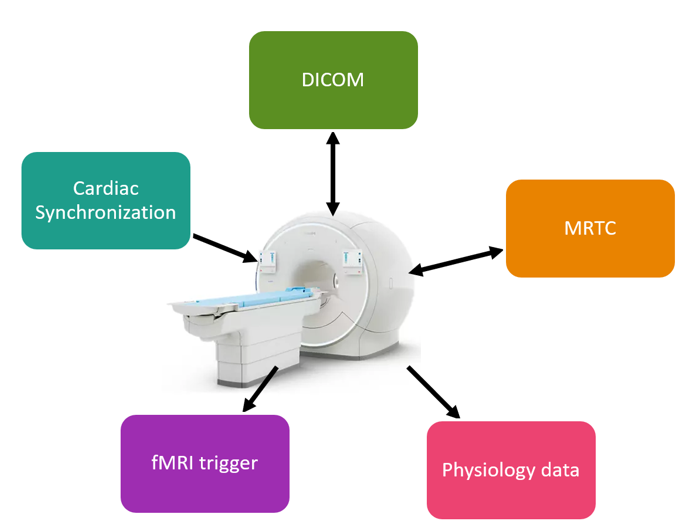
COMRADE encompasses the complete technical interface between the MRI scanner and the treatment device, including connection to physiology data and data sharing via nodes of the Digital Imaging and Communications in Medicine (DICOM) technical standard. The greatest complexity is around orchestrating the complete workflow during the treatment phase, where the treatment system is connected to the MRI system. Together, those systems should deliver one workflow for the patient and the medical staff. Both systems need to communicate via an agreed protocol using a software interface. This communication between the two systems is the heart of COMRADE and is called Magnetic Resonance Therapy Control (MRTC).
The MRTC interface is designed to be fast, reliable and secure. It supports communication between systems on different operating systems and programmed in different programming languages. The buildings blocks of MRTC are chosen in such a way that the treatment device can design their specific required workflow.
Integrating MR-guided treatment devices with the MR system offers three key benefits:
- Single episode of care: Integrating MR-guided treatment devices with the MR system is an important step towards realising a single episode of care with a single modality and integrated treatment devices.
- Better accuracy in treatment: Integrated device controls through a single harmonised, optimised interface results in better accuracy in treatment.
- Positioning Europe effectively in the MR-guided treatment market: The MR-guided treatment market is a growing market. For example, the transcranial magnetic stimulator (TMS) global market is expected to reach $1.70 billion in 2027 at an investment annual growth rate of 9.1% (1).
The integration of MR-guided treatment devices through COMRADE in SIGNET is an important step towards positioning European industry more effectively in the MR-guided treatment market. Specifically, it provides treatment device manufacturers with a quicker path to clinical evaluation and market adoption.
Because of the enormous promise and benefits, including beyond SIGNET, there is a growing interest in the MRTC/COMRADE interface among other therapy devices companies.
More information
https://itea4.org/project/signet.htmlhttps://www.linkedin.com/company/itea-signet/

